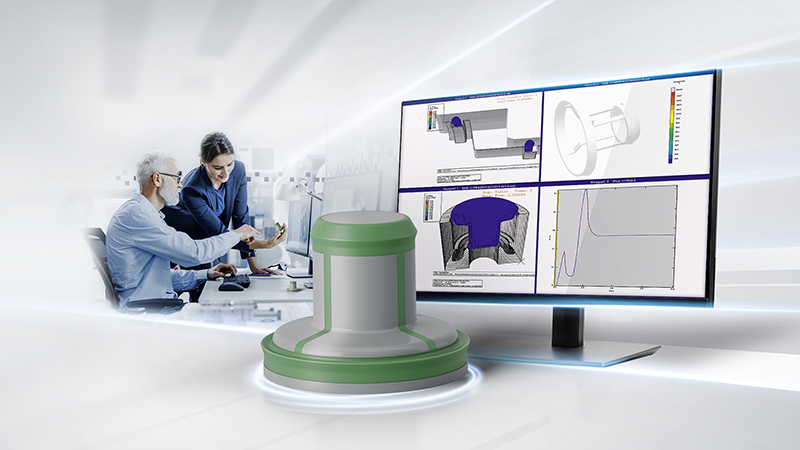
We are excited to announce the launch of our Rapid Development Center!
Trelleborg Medical Solutions created the Rapid Development Center in response to customer needs to accelerate time to market for new products and to reduce the production cost of parts already in market. This approach also improves the transition from design to serial production. The Rapid Development Center features dedicated equipment and personnel, enabling healthcare and medical customers to work with design, materials, process and quality experts throughout the entire life cycle of the project.
Learn more about our Rapid Development Center by exploring the links below!
Are you interested to hear even more about Trelleborg Medical Solutions?
Be the first to access new technical information from the Trelleborg experts about materials, manufacturing processes, and regulations impacting medical device design and production
Download our Brochures
Click the links below to download your very own copy of our latest brochures
- Trelleborg Medical Solutions – Your Partner for Life Changing Technologies
- Tubing, Hoses & Sheeting for Healthcare and Medical Applications
Pre-register for whitepapers
Select the whitepaper you’re interested in, complete the short registration form, and we’ll e-mail the PDF to you as soon as it becomes available. All will be released in 2021.
- Advanced Extrusion Technologies
- Drug-Eluting Devices: Introducing APIs to Silicone and Controlling Elution Rates
- How Multicomponent Molding Can Facilitate the Development of Next Generation Medical Devices
How Multicomponent Molding Can Facilitate the Development of Next Generation Medical Devices
Get notified of upcoming webinars
Select the webinar you’re interested in, provide your contact information, and we’ll e-mail the registration form to you as soon as registration opens.
- Drug-eluting silicone combination products
- Developments in biopharmaceutical processing
Access webinar recordings
Click the links to immediately access these webinar recordings.
- Tackle MDR requirements with your supplier’s material expertise
- Understanding Molding Complexity to Reduce Device Complexity
- Case studies in applying advanced extrusion technologies for medical device design
- Solving Complex Device Design Challenges through Partnership
Click here to have a Trelleborg Medical Solutions expert help you with your next project.
© Trelleborg Group. All rights reserved.